II. CONCEPT SELECTION
Step 11: Intellectual Property Basics
Intellectual Property – refers to creations of the mind, such as inventions; literary and artistic works; designs; and symbols, names, and images. Intellectual Property rights provide protection for creations and inventions, to enable creators and inventors to earn recognition and financial benefit from their work (Basic aspects of Intellectual Property, n.d.).
Criteria for obtaining a patent
There are three basic parameters for judging the patentability of an invention:
1. Utility – The invention must do something useful.
2. Novelty – It must be new with respect to other patents, products, or publicly available descriptions anywhere in the world (collectively known as the “prior art”).
3. Obviousness – The invention must not be obvious considering the prior art to someone of average skill and knowledge working in the given field (Zenios et al., 2010).
Step 12: Regulatory Basics
Because of the critically important role that regulatory issues play in the ultimate success of a new technology, understanding the regulatory pathway is imperative, even in the early stages of developing a device. In practice, innovators almost always employ an expert to actually write and manage their regulatory submissions. However, regulatory issues are so deeply embedded into product design and development that innovators need to understand regulatory processes and nomenclature to provide effective leadership in the biodesign innovation process (Zenios et al., 2010).
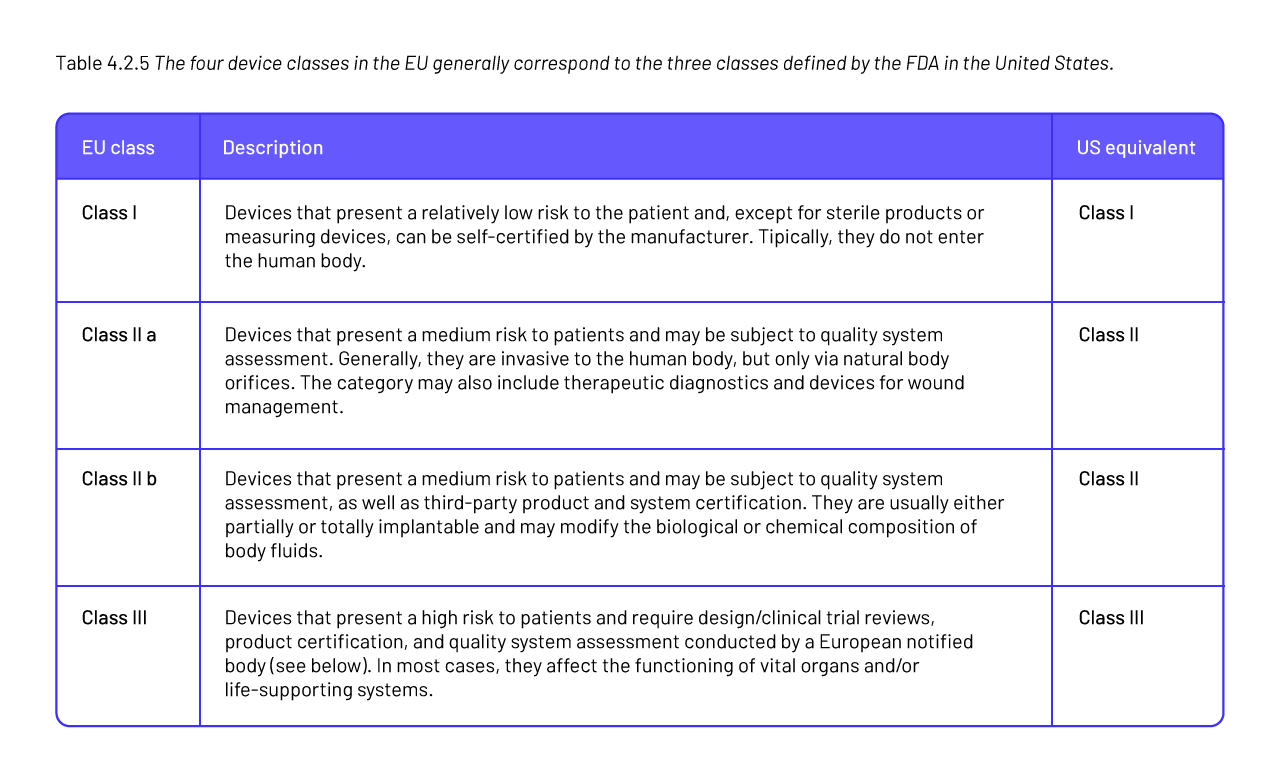
The US Food and Drug Administration (FDA) is a regulatory, scientific, and public health agency with a vast jurisdiction, overseeing products that account for roughly 25 percent of all consumer spending in the United States.1 Products under FDA jurisdiction include most foods (other than meat and poultry), human and animal drugs, therapeutic agents of biological origin, radiation-emitting products, cosmetics, animal feed, and, of course, medical devices (Regulatory Process | FDA, n.d.).
For Romania – ANMDM
For EU – EMA

(Zenios et al., 2010)
Step 13: Reimbursement Basics
The purpose of reimbursement analysis is to determine whether the existing healthcare payment infrastructure will accommodate a solution to a need, if such a solution becomes available. It addresses issues such as whether there will be adequate payment for the physicians using the solution, as well as for the facilities where patients would be treated. It also explores whether the coverage would be applicable to a large enough segment of the target market to make the development of the solution financially viable. If not, in today’s healthcare environment, the innovator must either anticipate slow (or no) adoption of the device or consider pursuing a change to the established payment infrastructure to accommodate the new solution (MEDIClever, n.d.; Reimbursement basics - PubMed, n.d.).
Sometimes the go to market strategy might not include reimbursement but this can only apply in very selected cases and can pose a serious challenge to sustainability of the project.

(Zenios et al., 2010)
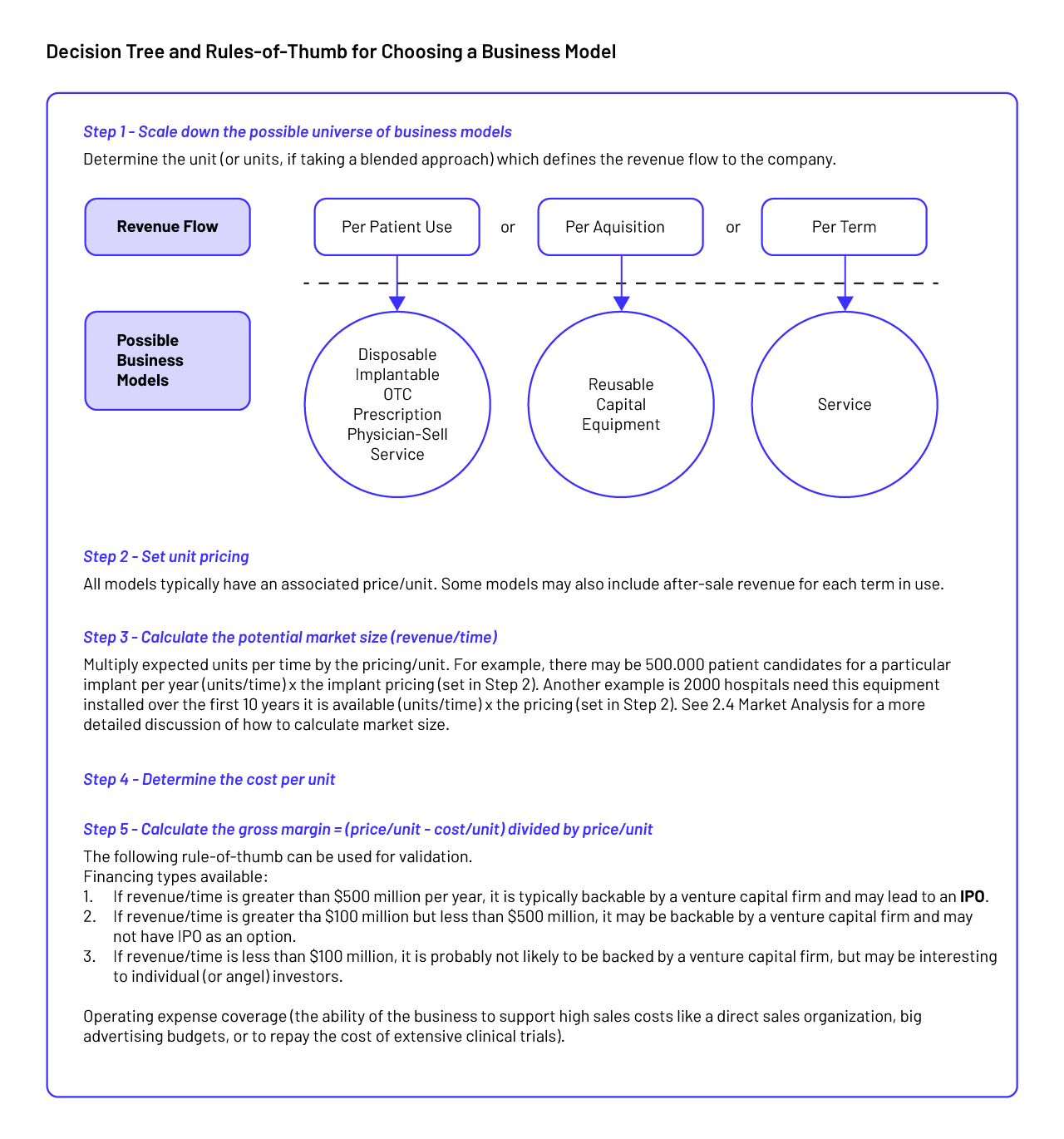
Step 14: Business Models
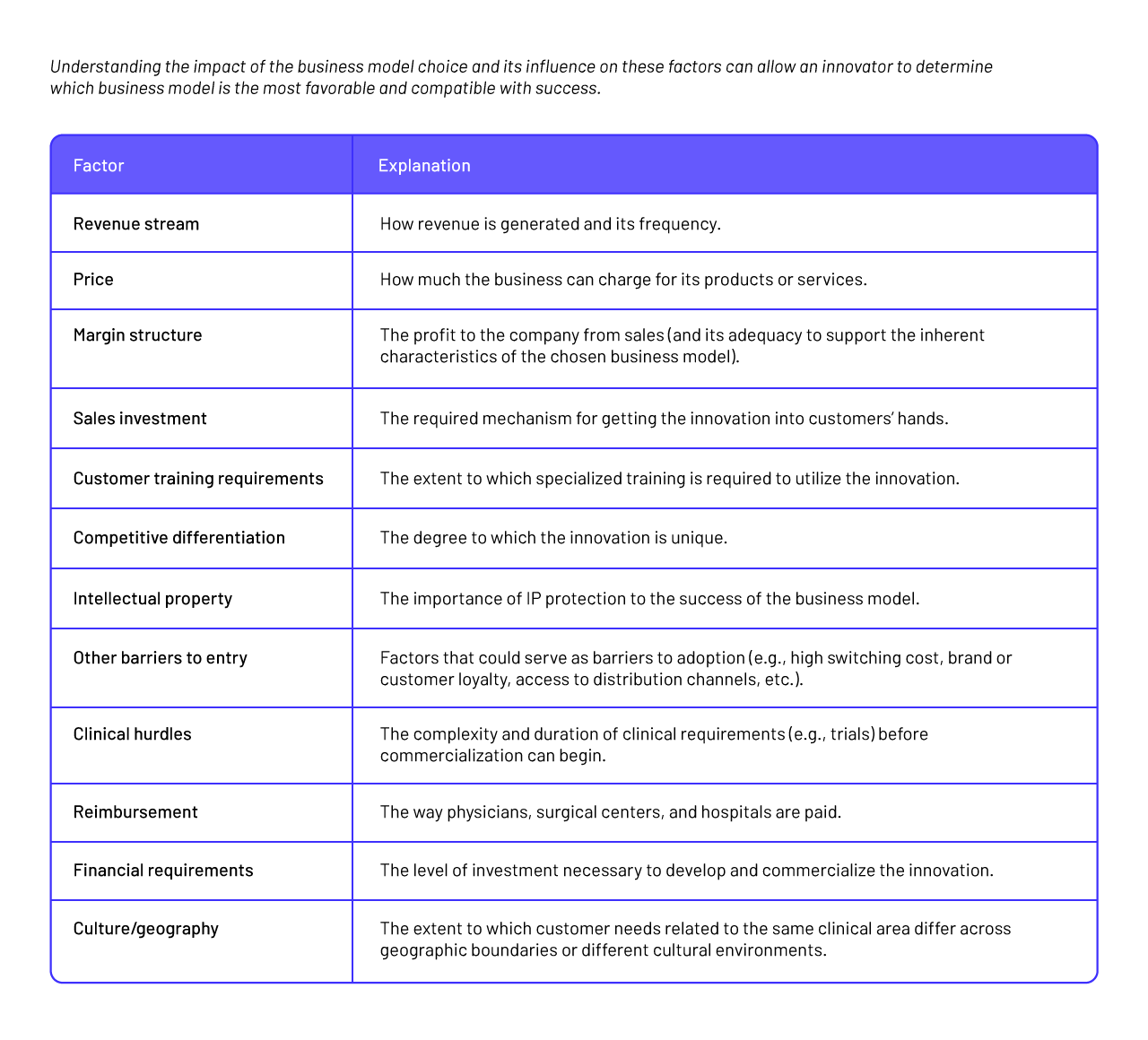
A business model broadly refers to how an offering (e.g., a product or service) is defined and the way it will generate revenue and deliver value to customers. In the medtech field, common business models include disposables, reusables, implantables, and capital equipment, although many others exist. With each of these models, offerings make money in different ways and, in the process, pose different challenges to the innovator in terms of how the company organizes its operations, resources, and processes. The business model also dictates, to some extent, how interactions with customers and other external stakeholders should be managed to achieve mutually beneficial results. Just as a company needs to assess the intellectual property (IP), regulatory, reimbursement, and technical feasibility of its ideas, it should evaluate the appropriate business model before selecting a final concept(Sharan et al., 2016).
(Zenios et al., 2010)
Types of business models:
- Disposable products: are those goods that are used and then discarded without being reused (paper examination gowns and stopcocks (used with intravenous tubing)).
- Reusable products: are multi-use products with a moderate lifespan, but their cost is orders of magnitude smaller than a capital equipment item ( scalpel holders, laparoscopic graspers, and endoscopes).
- Implantable products: are typically mid- ($1,000 to $5,000) to high-cost (>$5,000) items. An example of a mid-cost implantable device is a coronary artery stent.
- Capital equipment products: require customers to make a capital expenditure in order to obtain a technology that they will use repeatedly (magnetic resonance imaging (MRI) or computer tomography (CT) scanners, blood analytics equipment, or ultrasound machines)
- Service: the work performed by one person or group for the benefit of another.
- Fee-per-use: a fee-per-use business model can be appropriate for innovations that sit squarely at the intersection of products and services.
- Over-the-counter products: an over the counter (OTC) business model depends on patients’ ability to choose a treatment path and then acquire the product(s) for themselves. Over-thecounter products are more typically seen with drugs (e.g., Motrin®, Benadryl®), but can also include devices (e.g., steam machine for treating sinus congestion, home blood-pressure cuff devices, or glucose monitors).
- Prescription products: provide a classic example of prescription-based medical products. However, prescriptions can also be used for devices and combined with services (e.g., physical therapy), disposables (e.g., blood glucose monitoring testing strips, drug cartridges to a delivery system), and hardware (e.g., nerve stimulator pain control units, certain inhalers).
- Physician-sell products: are those treatments that are sold directly through physicians (Zenios et al., 2010).

(Zenios et al., 2010)
Step 15: Prototyping
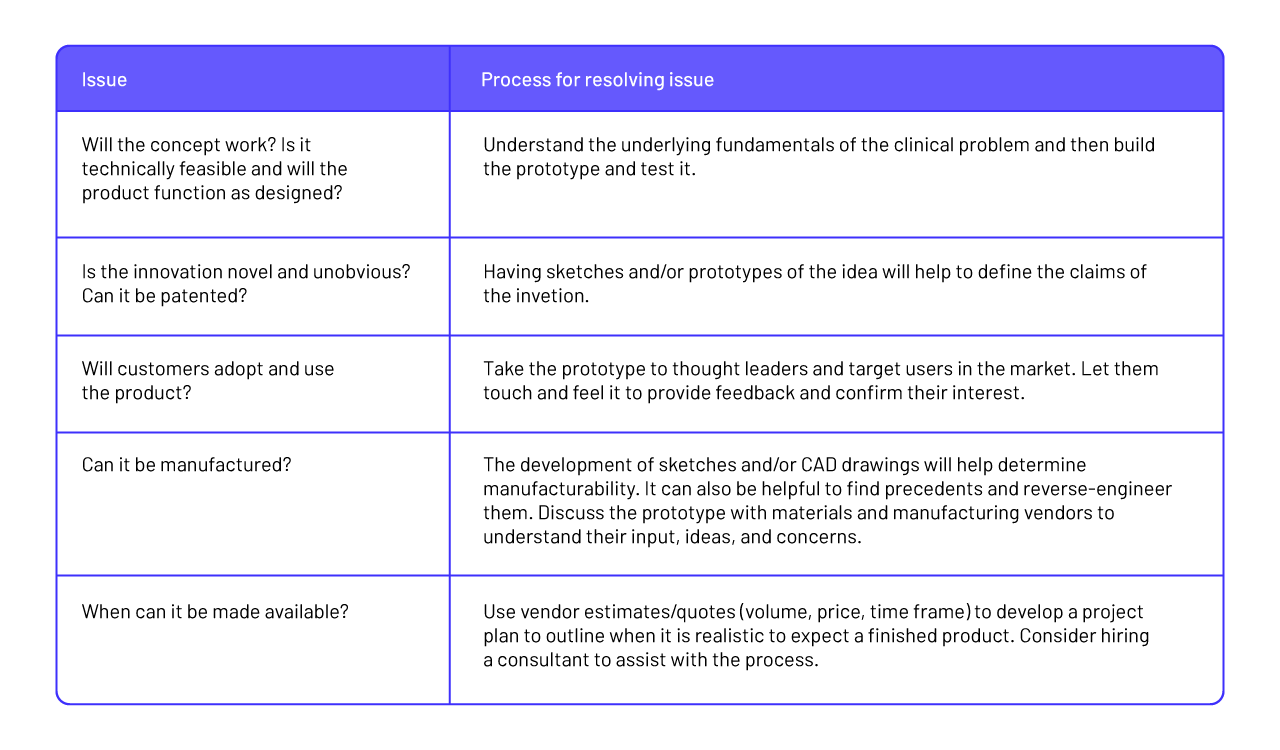
The goal of prototyping is to translate a promising concept from an idea into a rudimentary design, and then into a working form. Prototyping is an essential step through which the innovator learns about functionality, explores features, gathers preliminary feedback from target users, and answers questions that can only be resolved through the manifestation of the design. Prototyping plays a role at multiple stages of the biodesign innovation process. Early on, prototyping can be performed quickly and inexpensively to help the innovator evaluate multiple solution ideas against more specific design criteria before deciding on a final concept. As the innovator moves forward, prototype requirements, designs, and models become more advanced. These more robust prototypes are used to test functionality and features, often in conjunction with tissue and animal testing. Near final prototypes that meet design requirements are used in gathering data for quality documentation and preparation for manufacturing (Zenios et al., 2010).

(Zenios et al., 2010)
Step 16: Final Concept Selection
The purpose of final concept selection is to use everything that has been learned about all concepts under consideration to choose the one that will be brought forward into development. While a final concept will continue to be refined, tested, iterated, and improved during development and implementation, the innovator will focus on pursuing that single concept rather than dividing his/her attention across multiple potential solutions. While some experienced innovators are able to make this decision based on gut instinct, most benefit from a more formalized process, such as the Pugh concept selection method (Zenios et al., 2010).

(Zenios et al., 2010)